Primary efficacy endpoint was the proportion of subjects with short duration atrial fibrillation (3 hours to 7 days) who had a treatment-induced conversion of atrial fibrillation to sinus rhythm for a minimum duration of one minute within 90 minutes of first exposure to study drug. Efficacy was studied in a total of 390 haemodynamically stable adult patients with short duration atrial fibrillation including patients with hypertension (40.5%), ischaemic heart disease (12.8%), valvular heart disease (9.2%) and CHF (10.8%). In these studies treatment with BRINAVESS effectively converted atrial fibrillation to sinus rhythm as compared with placebo (see Table x). Conversion of atrial fibrillation to sinus rhythm occurred rapidly (in responders the median time to conversion was 10 minutes from start of first infusion) and sinus rhythm was maintained through 24 hours (97%). The vernakalant dose recommendation is a titrated therapy with two possible dose steps. In the performed clinical studies, the additive effect of the second dose, if any, cannot be independently established.
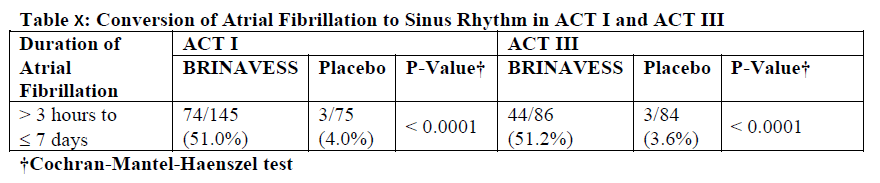
BRINAVESS was shown to provide relief of atrial fibrillation symptoms consistent with conversion to sinus rhythm.
No significant differences in safety or effectiveness were observed based on age, gender, use of rate control medications, use of antiarrhythmic medications, use of warfarin, history of ischaemic heart disease, renal impairment or expression of the cytochrome P450 2D6 enzyme.
Treatment with BRINAVESS did not affect the response rate to electrical cardioversion (including the median number of shocks or joules required for successful cardioversion) in cases when attempted within 2 to 24 hours of study medicine administration.
Conversion of atrial fibrillation in patients with longer-duration atrial fibrillation (> 7 days and ≤ 45 days) assessed as a secondary efficacy endpoint in a total of 185 patients did not show statistically significant differences between BRINAVESS and placebo.
References:
-
עלון לרופא עמודים 8-9

