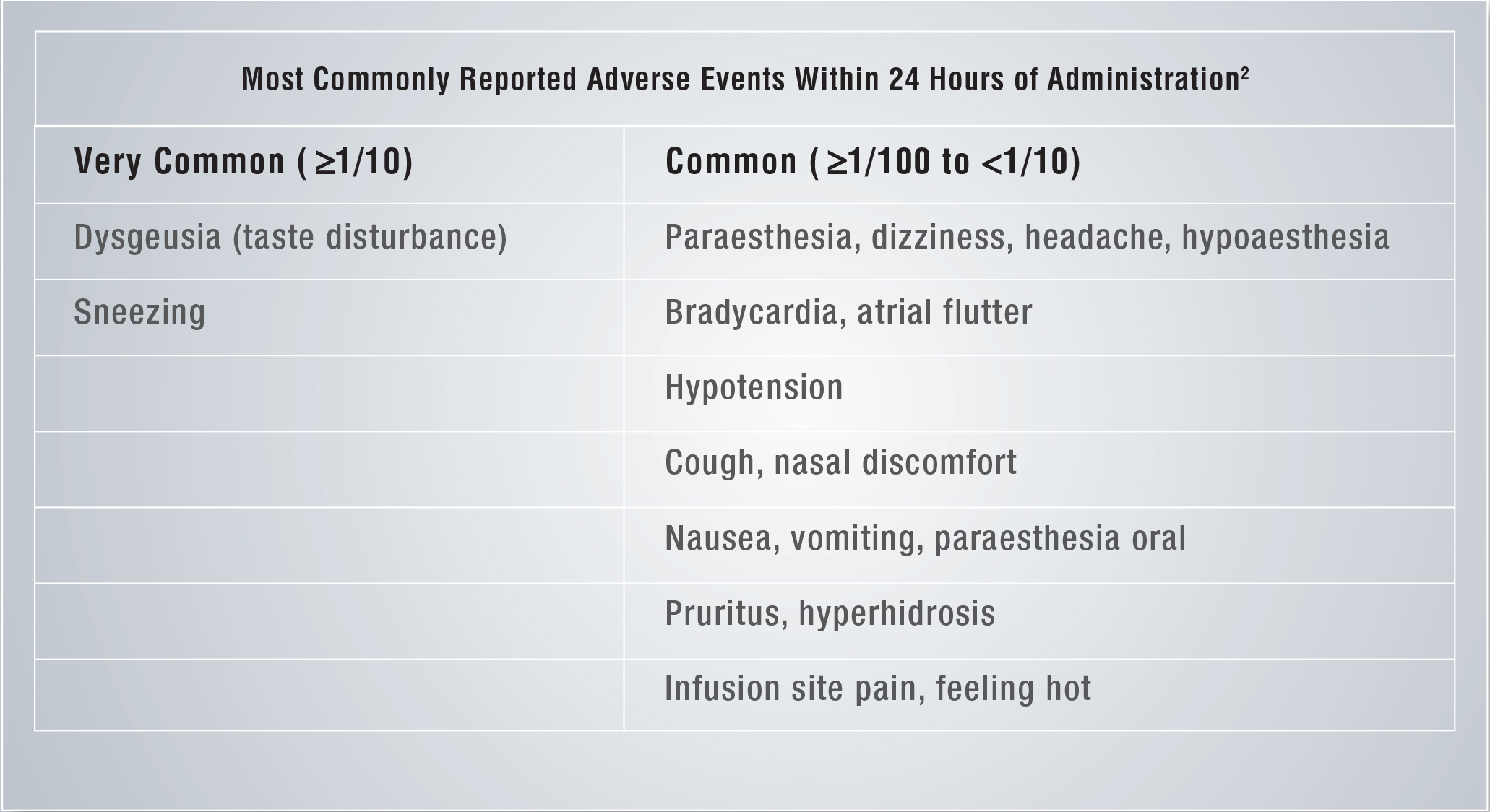
Brinavess- Reported Adverse Events Within 24 hours of Administration¹
Frequencies are defined as: very common (≥ 1/10); common (≥ 1/100 to < 1/10); uncommon (≥ 1/1,000 to < 1/100); rare (≥ 1/10,000 to < 1/1,000) and very rare (< 1/10,000), not known (cannot be estimated from the available data)
BRINAVESS is contraindicated in:¹
• Patients with hypersensitivity to the active substance or to any of the excipients.
• Patients with severe aortic stenosis, patients with systolic blood pressure <100 mm Hg, and patients with heart failure class NYHA III or NYHA IV.
• Patients with prolonged QT at baseline (uncorrected >440 msec), or severe bradycardia, sinus node dysfunction, or second-degree or third-degree heart block in the absence of a pacemaker.
• Patients who have received intravenous rhythm control antiarrhythmics (class I and class III) within 4 hours prior to administration of BRINAVESS. In addition, the use of intravenous rhythm control antiarrhythmics (class I and class III) is contraindicated within 4 hours after administration of BRINAVESS.
• Patients with acute coronary syndrome (including myocardial infarction) within the last 30 days.
NYHA=New York Heart Association
No significant difference in safety or effectiveness was observed based on:¹
Short half-life¹
The mean elimination half-life of BRINAVESS in patients was:
- Approximately 3 hours in extensive metabolisers of CYP2D6
- Approximately 5.5 hours in poor metabolisers of CYP2D6
Resumption or initiation of oral maintenance antiarrhythmic therapy can be considered starting 2 hours after BRINAVESS administration
Acute exposure is not significantly influenced by:¹
• Gender, history of CHF, or renal impairment
• Concomitant administration of ß-blockers and other medications, including warfarin, metoprolol, furosemide, and digoxin
• No dose adjustment of BRINAVESS is required for these conditions, or for age, serum creatinine or CYP2D6 metaboliser status
• No dose adjustment for special populations
No dose adjustment needed based on age, serum creatinine, or renal or hepatic impairment
• BRINAVESS is dosed by patient body weight, with a maximum calculated dose based upon 113 kg
–For patients weighing ≥113 kg:
Do not exceed the maximum initial dose of 339 mg; do not exceed the maximum second infusion of 226 mg
–Cumulative doses of >5 mg/kg should not be administered within 24 hours
Safety
Hypotension
Hypotension can occur in a small number of patients (vernakalant 7.6%, placebo 5.1%). Hypotension typically occurs early, either during the infusion or early after the end of the infusion, and can usually be corrected by standard supportive measures. Uncommonly, cases of severe hypotension have been observed. Patients with congestive heart failure (CHF) have been identified as a population at higher risk for hypotension. The patient is required to be monitored for signs and simptoms of a sudden decrease in blood pressure or heart rate for the duration of the infusion and for at least 15 minutes after the completion of infusion.
Congestive Heart Failure (CHF)
- In patients without a history of CHF, rates of ventricular arrhythmias were similar between BRINAVESS and placebo (3.2% vs 3.6%, respectively)
- Patients with a history of CHF showed a higher incidence of ventricular arrhythmia in the first two hours post dose (7.3% for BRINAVESS compared to 1.6% in placebo).
These arrhythmias typically presented as asymptomatic, monomorphic, non-sustained (average 3-4 beats) ventricular tachycardias.
BRINAVESS should be used cautiously in haemodynamically stable patients with CHF functional classes NYHA I to II. It is not recommended that BRINAVESS is used in patients with previously documented LVEF ≤ 35% because of lack of data in this patient group.
BRINAVESS is contraindicated in patients with severe aortic stenosis, patients with systolic blood pressure < 100 mm Hg, and patients with heart failure class NYHA III and NYHA IV
Atrial Flutter
No patient with AFL following treatment with BRINAVESS developed 1:1 atrioventricular conduction
- AF patients receiving BRINAVESS have a higher incidence of converting to atrial flutter within the first 2 hours post-dose (10.0% vs 2.5% with placebo)
- With continuation of the medicine infusion as recommended, the majority of these patients continue to convert to SR. in the remaining patients, electrical cardioversion can be recommended
Use of AADs (anti-arrhythmic drugs) prior to or after BRINAVESS
BRINAVESS cannot be recommended in patients previously administered intravenous AADs (class I and III) 4-24 hours prior to vernakalant due to lack of data. BRINAVESS should not be administered in patients who received intravenous AADs (class I and III) within 4 hours prior to vernakalant.
BRINAVESS should be used with caution in patients on oral AADs (class I and III), due to limited experience.
There is limited experience with the use of intravenous rhythm control anti-arrhythmics (class I and class III) in the first 4 hours after BRINAVESS administration, therefore these agents should not be used within this period.
Valvular Heart Disease
In patients with valvular heart disease, there was a higher incidence of ventricular arrhythmia events in vernakalant patients. These patients should be monitored closely.
Other Diseases and Conditions not Studied
Within the clinical development program, BRINAVESS has been administered to patients with an uncorrected QT < 440 msec without an increased risk of torsade de pointes
Furthermore, BRINAVESS has not been evaluated in patients with clinically meaningful valvular stenosis, hypertrophic obstructive cardiomyopathy, restrictive cardiomyopathy, or constrictive pericarditis and its use can not be recommended in such cases. There is limited experience with BRINAVESS in patients with pacemakers.
As the clinical trial experience in patients with advanced hepatic impairment is limited, vernakalant is not recommended in these patients.
This medicinal product contains approximately 1.4 mmol (32 mg) sodium in each 200 mg vial. Each vial of 500 mg contains approximately 3.5 mmol (80 mg) of sodium.
This should be taken into consideration by patients on a controlled sodium diet.