VEOZA achieved clinically meaningful VMS reductions and was evaluated for safety for up to a year1
Study Design
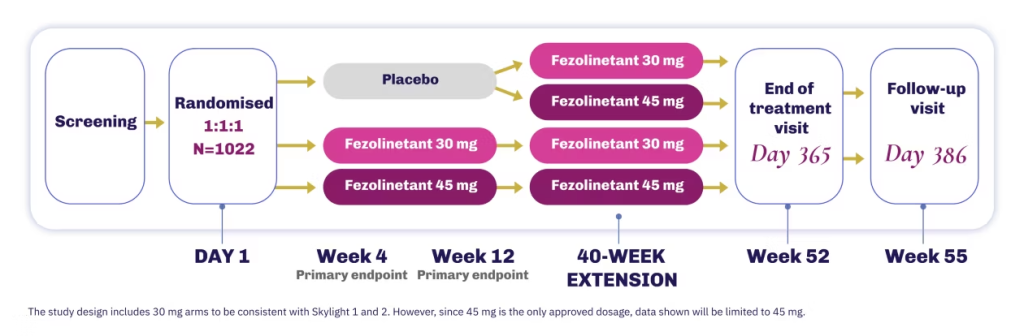
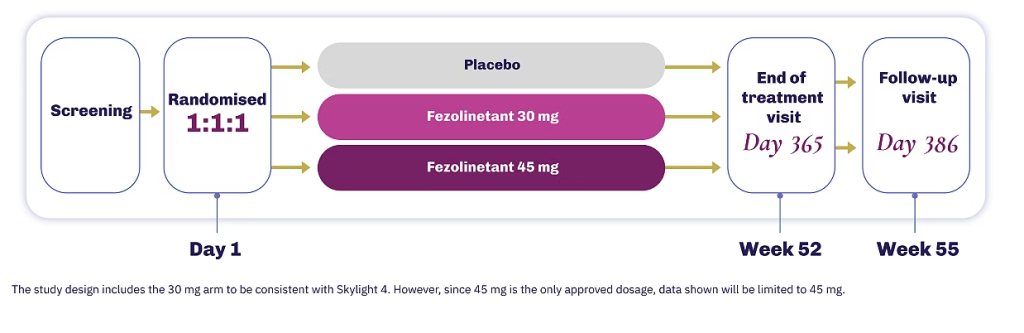
The efficacy of VEOZA was evaluated in two 12-week, randomised, placebo-controlled, double-blind Phase 3 studies, followed by a 40-week uncontrolled extension treatment period1
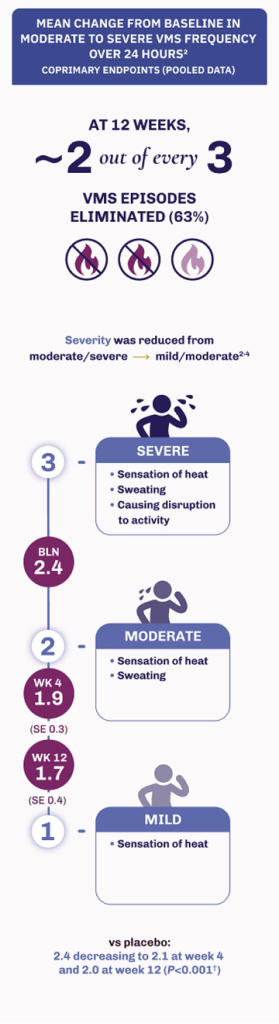
Coprimary endpoints: Mean change from baseline in moderate to severe VMS frequency and severity1
*Clinically meaningful is defined as a reduction in ≥2 hot flushes per day versus placebo.1
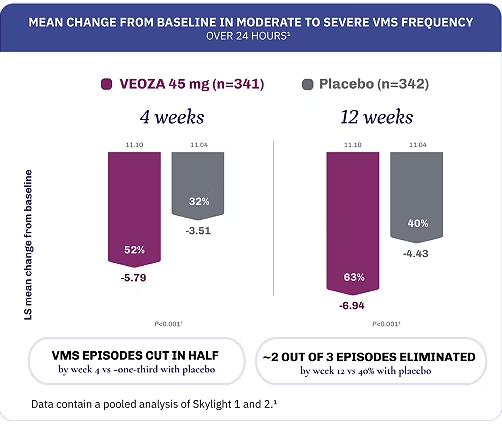
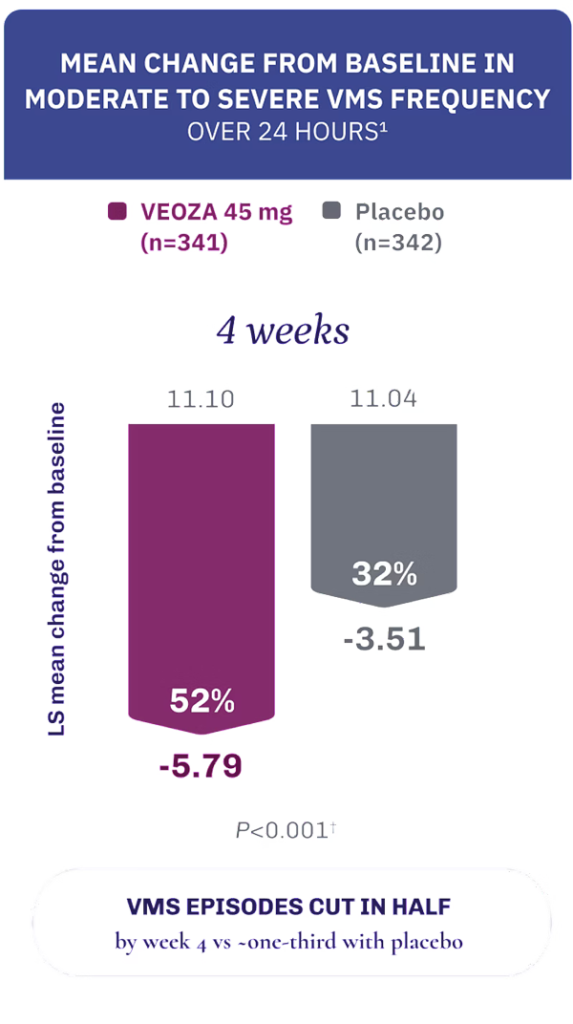
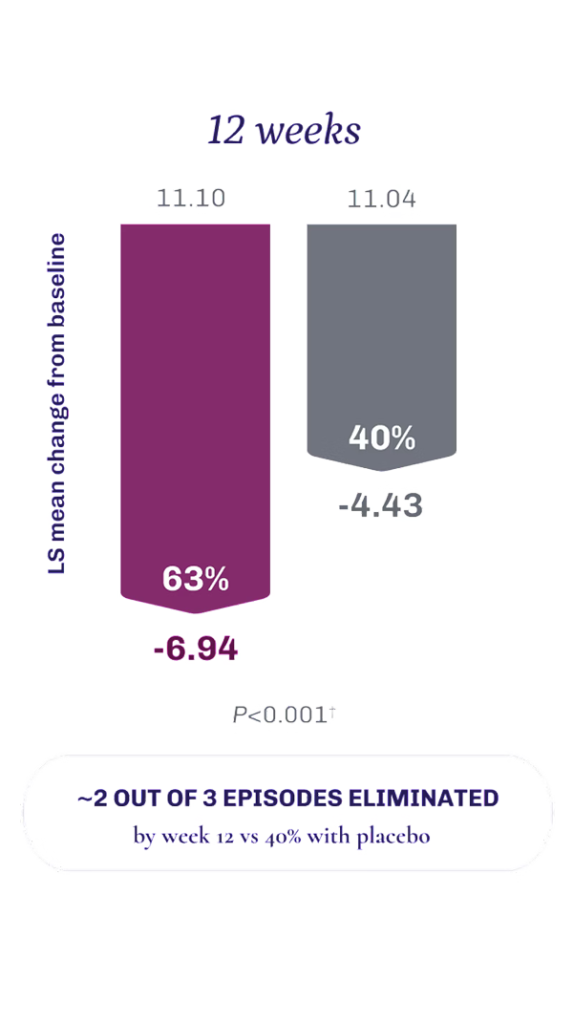
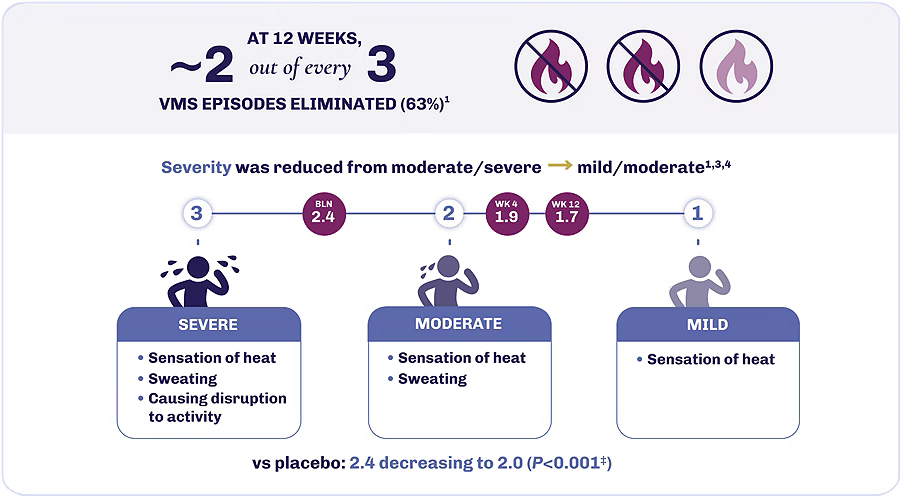
Achieve fewer and less severe VMS episodes with VEOZA1
VEOZA provides statistically significant relief from the number and severity of daily hot flushes and night sweats1
FREQUENCY: Measured as a daily mean and analyzed as weekly average.1
LS mean: Least squares mean estimated from a mixed model for repeated measures analysis of covariance.1
†Statistically significantly superior compared to placebo at the 0.05 level with multiplicity adjustment.1
Reduction in severity of VMS episodes1
BLN: Baseline.
Data contain a pooled analysis of Skylight 1 and 2.
‡Statistically significantly superior compared to placebo at the 0.05 level with multiplicity adjustment.1

Now there’s VEOZA, with results to help your patients continue activity with less sweat and disruption1,3,4
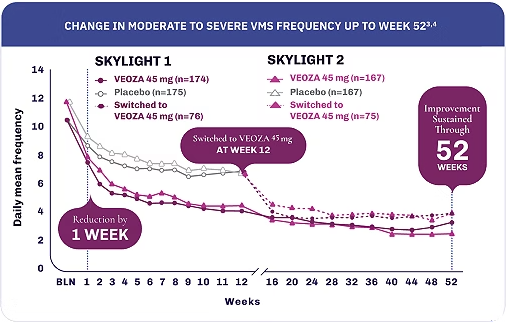
Relief that works fast and lasts3,4
Mean change in frequency of VMS from baseline to each week up to week 12 was a secondary endpoint and was not adjusted for multiplicity.3,4
Mean change in the frequency of VMS from baseline to each visit in the extension period was an exploratory endpoint. Assessments after the 12-week placebo-controlled period were descriptive only.4,5
VEOZA was studied for safety and tolerability for 1 year1
Skylight 1 & 2
Skylight 4
Adverse reactions for VEOZA 45 mg
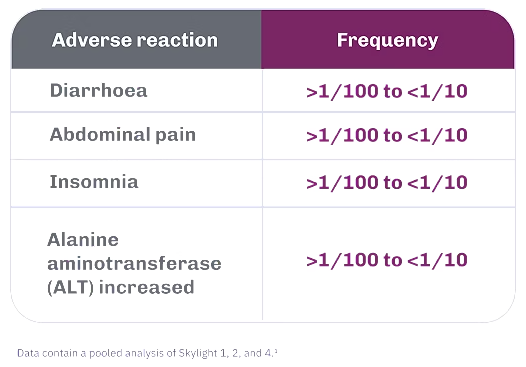
- Across the Phase 3 studies, the most common adverse reactions (≥3%) with VEOZA 45 mg were diarrhoea (3.2%) and insomnia (3.0%). The most frequent adverse reactions leading to dose discontinuation with VEOZA were alanine aminotransferase (ALT) increased (0.3%) and insomnia (0.2%)
- There were no serious adverse reactions reported at an incidence greater than 1% across the total study population
VEOZA was assessed for endometrial safety
In the long-term safety data, VEOZA was evaluated for tolerability and endometrial safety compared to placebo over 52 weeks1
- 1 case of endometrial adenocarcinoma was observed
- Endometrial biopsy assessments did not identify an increased risk of endometrial hyperplasia or malignancy according to prespecified criteria for endometrial safety
- Transvaginal ultrasound did not reveal increased endometrial thickness
REFERENCES: 1. VEOZA [Veoza Israeli SmPC] 2. Thurston RC. Vasomotor symptoms. In: Crandall CJ, Bachman GA, Faubion SS, et al., eds. Menopause Practice: A Clinician’s Guide. 6th ed. Pepper Pike, OH: The North American Menopause Society, 2019:43-55. 3. Lederman S, Ottery FD, Cano A, et al. Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet 2023;401(10382):1091-102. 4. Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate to severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab (Epub) 02-03-2023. 5. Astellas. VEOZA. Data on file.